Kwanan nan, FDA ta fitar da ƙa'idodin ƙarshe na jeri na kayan kwalliya da samfuran, kuma sun ƙaddamar da sabon tashar kayan shafawa mai suna 'Cosmetic Direct'. Kuma, FDA ta sanar da buƙatun wajibai don rajistar kayan aikin kwaskwarima da jeri na samfur daga Yuli 1, 2024, don tabbatar da kasuwancin da aka tsara suna da isasshen lokacin shirya da ƙaddamar da bayanai.
1. Dokoki
1) Sabunta Dokar Kayayyakin Kayan Aiki na 2022, (MoCRA)
2) Dokar Abinci, Magunguna da Kayan kwalliya ta Tarayya (Dokar FD&C)
3) Dokar Marufi da Lakabi na Gaskiya (FPLA)
2. Iyakar aikace-aikace
A cewar dokar Amurka, ana ayyana kayan kwalliya azaman abubuwan da ake shafa, baza, fesa, ko akasin haka a jikin ɗan adam don tsaftacewa, ƙawata, haɓaka kyan gani, ko canza kamanni.
Musamman ma ya hada da man shafawa na fata, turare, lipstick, gogen farce, kayan kwalliyar ido da fuska, gogewar shamfu, perm, rini na gashi da deodorant, da duk wani abu da ake amfani da shi azaman kayan kwalliya. Sabulu baya na kayan kwalliya.
3. Rarrabewa
A cewar MoCRA, FDA na kayan shafawa na Amurka yana rarraba kayan kwalliya zuwa nau'ikan masu zuwa:
-Kayan jarirai: ciki har da shamfu na jarirai, foda talcum na kula da fata, kirim na fuska, mai da ruwa.
-Kayan wanka: ciki har da gishirin wanka, mai, magani, wakilin kumfa, gel ɗin wanka, da sauransu.
-Kayan gyara ido: kamar fensir gira, gashin ido, inuwar ido, wanke ido, kayan shafa ido, baki baki, da sauransu.
Kayan shafawa tare da sakamako na musamman, irin su anti wrinkle, whitening, asarar nauyi, da sauransu, suna buƙatar yin rijista azaman magungunan OTC a lokaci guda. Ya kamata a lura cewa waɗannan sabbin ka'idoji sun shafi kayan kwalliyar da ake fitarwa zuwa kasuwannin Amurka.
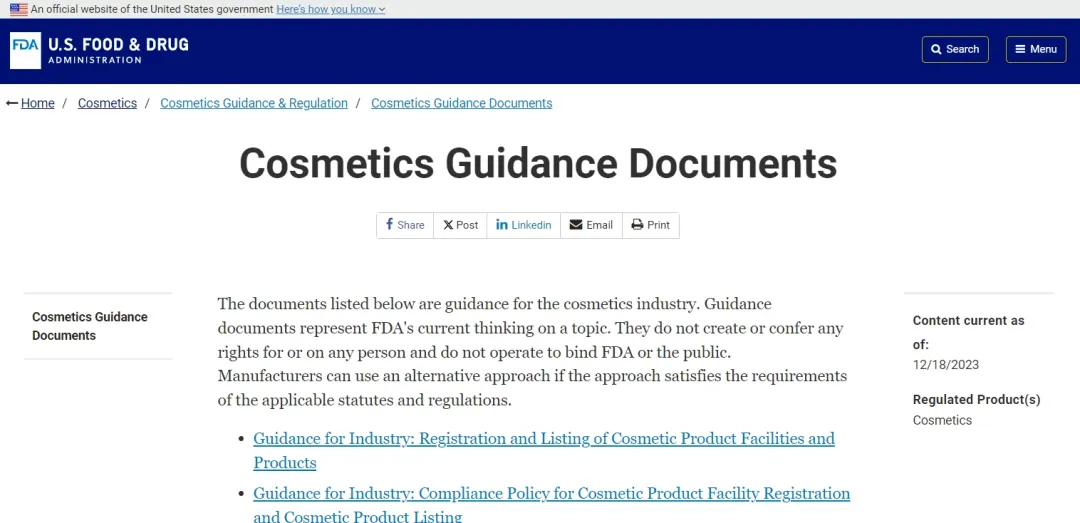
FDA rajista
MoCRA ba kawai ta ƙara waɗannan sabbin buƙatu masu zuwa ba, gami da kafa tsarin da ke da alhakin kayan kwalliya, bayar da rahoton wajaba game da munanan halayen, yarda da Kyawawan Kyawawan Ayyukan Masana'antu (GMP), rajistar kayan aikin masana'anta da rajistar samfuran samfuran, samar da isassun takaddun aminci, amma Hakanan yana buƙatar alamar alamar tare da bayanin mutumin da ke da alhakin, ainihin allergens, yin amfani da ƙwararrun bayanan samfur, haɓakawa da sakin hanyoyin gano asbestos kayan kwalliyar da ke dauke da talcum foda, da ƙimar haɗarin aminci da fitar da gwajin dabba na PFAS a cikin kayan kwalliya.
Kafin aiwatar da MOCRA, masana'antun kayan kwalliya/masu kayan kwalliya na iya yin rijistar wuraren masana'antar su tare da FDA ta hanyar Shirin Rijistar Kayan Kayan Kayan Kayan Kaya na Amurka (VCRP), kuma FDA ba ta da buƙatu na wajibi don wannan.
Amma tare da aiwatar da MOCRA da kuma ƙarshen wajabcin gabatowa, duk kamfanonin da ke siyar da kayan kwalliya a Amurka dole ne su yi rajistar masana'antunsu tare da FDA kuma su sabunta bayanan rajistar su duk bayan shekaru biyu, gami da suna, bayanin lamba, da sauransu. Kayayyakin da ke wajen Amurka. Ana kuma buƙatar jihohi don samar da bayanai da bayanan tuntuɓar wakilai a cikin Amurka. Hakanan akwai wasu ƙarin bayanai waɗanda ke buƙatar cikewa, kamar bayanan kamfani na iyaye, nau'in kamfani, hotuna marufi, hanyoyin haɗin yanar gizon samfur, ko ƙwararrun kayan kwalliya ne, lambar Dun&Bradstreet na mutumin da ke da alhakin, da sauransu. Ba wajibi ba ne a cika. In. Abubuwan kayan kwalliyar da suka wanzu dole ne su yi rajista tare da FDA a cikin shekara guda bayan an ba da sabbin ka'idoji, kuma lokacin rajista don sabbin kayan kwaskwarima yana cikin kwanaki 60 na shiga ciki. sarrafa kayan kwalliya da samarwa.
Lab Gwajin BTF, kamfaninmu yana da dakunan gwaje-gwaje masu dacewa da lantarki, ka'idojin aminci Laboratory, Laboratory mitar rediyo, dakin gwaje-gwaje na baturi, dakin gwaje-gwajen sinadarai, dakin gwaje-gwaje na SAR, dakin gwaje-gwaje na HAC, da sauransu. Mun sami cancanta da izini kamar CMA, CNAS, CPSC, VCCI. da dai sauransu. Kamfaninmu yana da ƙwararrun ƙwararrun ƙwararrun ƙwararrun ƙwararrun ƙwararrun ƙwararrun ƙwararrun ƙwararrun ƙwararrun ƙwararrun ƙwararrun ƙwararrun ƙwararrun injiniyoyi, waɗanda za su iya taimaka wa kamfanoni su magance matsalar. Idan kuna da gwajin da suka dace da buƙatun takaddun shaida, zaku iya tuntuɓar ma'aikatan Gwajin mu kai tsaye don samun cikakkun bayanan farashi da bayanan sake zagayowar!

Rahoton gwaji na FDA
Lokacin aikawa: Agusta-21-2024